Tunge Forlengelese FAQ and Tygon: Difference between pages
(Page conversion via llm-mediawiki-rev -jwm) |
(Page conversion via llm-mediawiki-rev -jwm) |
||
| Line 1: | Line 1: | ||
[[File: | {| style="text-align: right;" | ||
|[[File:Tygon1.jpg|thumb|right|tygon in a [[Surface_piercing|surface piercing]]]] | |||
|[[File:Tygon2.jpg|thumb|right|tygon in an [[Industrial_piercing|industrial piercing]]]] | |||
|} | |||
'''Tygon''' is a brand name silicone tubing made by: [http://www.tygon.com/ Saint-Gobain Performance Plastics]. It is highly inert and extremely flexible plastic tubing used for [[Surface_Piercing|surface]] and other [[Piercing|piercings]]. It usually has to be replaced every 3-12 months, if not sooner. | |||
There are many forms of silicone tubing so the client and piercer must be fully understanding of the type of material and quality they are using when in relation to body piercing procedures. The brand name, "''Tygon''", has only a couple forms that meet Bio-Compatibility standards which usually is when the company states the material is fully characterized to '''ISO 10993''' and FDA guidelines for biocompatibility. So piercers and clients should investigate into exactly what form of silicone tubing they are obtaining and to make sure it meets these standards and guidelines, before using it or having it used in relation to body piercing procedures. | |||
== | == See Also == | ||
* [[Jewelry]] | |||
* [[PTFE]] | |||
* [[Nylon]] | |||
* [[ | |||
* [[ | |||
* [[ | |||
Latest revision as of 21:39, 25 September 2023
 tygon in a surface piercing |
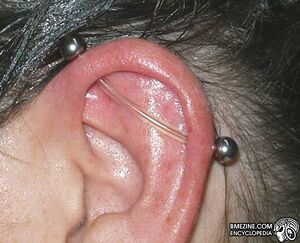 tygon in an industrial piercing |
Tygon is a brand name silicone tubing made by: Saint-Gobain Performance Plastics. It is highly inert and extremely flexible plastic tubing used for surface and other piercings. It usually has to be replaced every 3-12 months, if not sooner.
There are many forms of silicone tubing so the client and piercer must be fully understanding of the type of material and quality they are using when in relation to body piercing procedures. The brand name, "Tygon", has only a couple forms that meet Bio-Compatibility standards which usually is when the company states the material is fully characterized to ISO 10993 and FDA guidelines for biocompatibility. So piercers and clients should investigate into exactly what form of silicone tubing they are obtaining and to make sure it meets these standards and guidelines, before using it or having it used in relation to body piercing procedures.